



MORE TRANSPARENCY, HIGHER EFFICIENCY AND LOWER COST
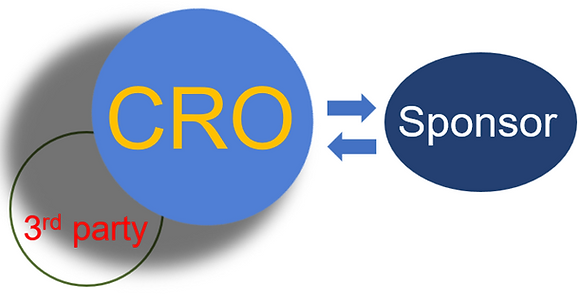

Traditional Business Model
Optimal Business Model


O U R F A C I L I T I E S




-
Ambient Room (15 ~ 25 ℃)
-
Cold Room (2 ~ 8 ℃)
-
Freezer Storage Room (-20 ℃ & -80 ℃)
-
24/7 Temperature and Humidity Monitoring System
-
Inventory Management System
-
Access Control Security System
-
Full Area Coverage Camera System
-
Fireproof Filing Cabinet
-
1.5M kW Backup Generator


Q U A L I T Y A S S U R A N C E
-
Registered with US FDA under FEI 3020085632
-
Successful EU QP Declaration
-
Strict SOPs in compliance with FDA, EMA, NMPA, TFDA, etc.
-
Inventory management system
-
Senior management team has an average of 30+ years of experience in top pharmaceuticals worldwide
-
A functional and effective team
-
Cloud and hardcopy data storage



O U R T E A M

Over 20 years of experience in pharma R&D and BD at Eli Lilly, Merck, and GenScript
Successfully led ACTS and completed over 50 global clinical trials (Phases I-III)
Provided RLD/Comparators to more than 400 pharma clients

Over 40 years experience in Pharmaceutical QA, Clinical QA, and regulatory compliance including 26 years at Pfizer as a senior leader
Provided regulatory and compliance support to clinical and commercial internal and 3ʳᵈ party sites around the globe
Directed a team of internationally based auditors, assessors, and QA support staff

